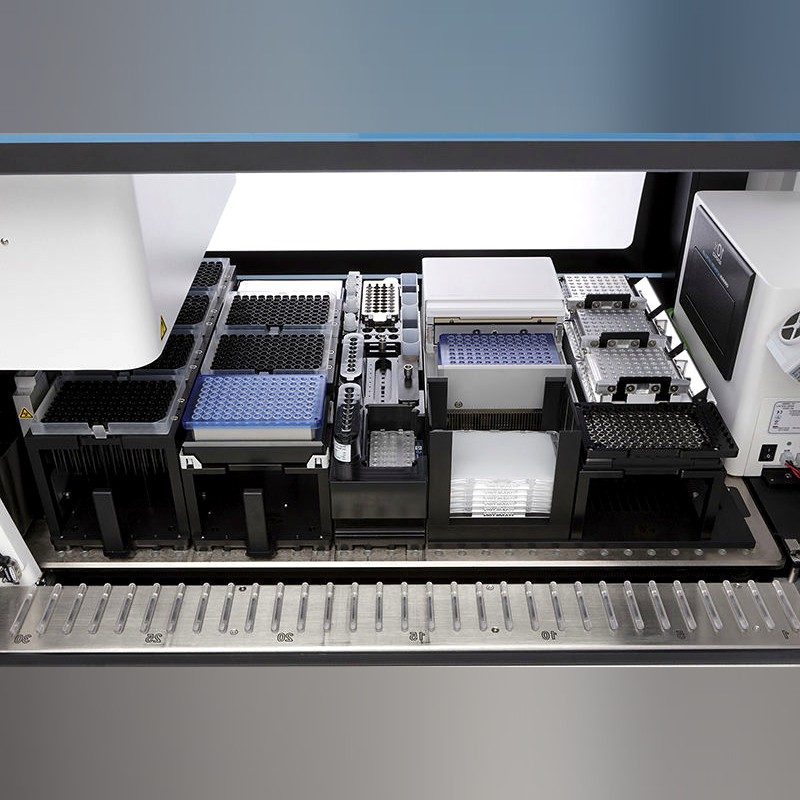
Automatic Sample Preparation System
Chromium Connect Automated Single Cell Library Preparation System
10xgenomics
LaboratoryCellFor Sequencing
Request a Quote
H2: Automated Sample Preparation System for High-Throughput Single Cell Analysis
- Company: Gold Medicals
- Application Areas: Genomics, Immunology, Oncology, Cell Biology, Translational Research
- Key Features:
- Consistent Results: Minimize variability in single cell data to achieve reproducible and reliable outcomes across experiments, users, and laboratories.
- Maximum Lab Productivity: Enable walk-away operation for single cell workflows, freeing up valuable hands-on time for researchers to focus on other critical tasks.
- Integrated and Validated Workflow: Automate the entire process of single cell partitioning, barcoding, and library preparation using a single optimized instrument platform.
- Multi-Assay Compatibility: Support comprehensive single cell analysis through gene expression profiling and immune receptor characterization in a unified system.
- One-Day Automated Workflows: Generate sequencing-ready libraries from sample input to final output within a single day, with flexible modular options for workflow customization.
- Modular Workflow Flexibility: Automate library construction starting from cDNA manually prepared on Chromium X/iX or Controller systems, or from prior Connect runs.
- World-Class Technical Support: Access expert on-site instrument installation and remote troubleshooting assistance to ensure optimal performance and system uptime.
H2: Clinical and Research Advantages
- Enables standardized automation of single cell workflows including partitioning, barcoding, and library preparation for consistent data generation across diverse research environments.
- Facilitates high-resolution exploration of cellular heterogeneity, identification of novel biomarkers, and discovery of therapeutic targets through automated 3’ gene expression analysis.
- Designed to support scalable, reproducible studies in multi-site research initiatives with minimal inter-run and inter-user variability.
H2: Compliance and Certification
- Compliant with ISO 13485 standards for medical device quality management systems
- CE marked for use in the European Economic Area
- FDA 510(k) cleared for diagnostic applications involving single cell analysis